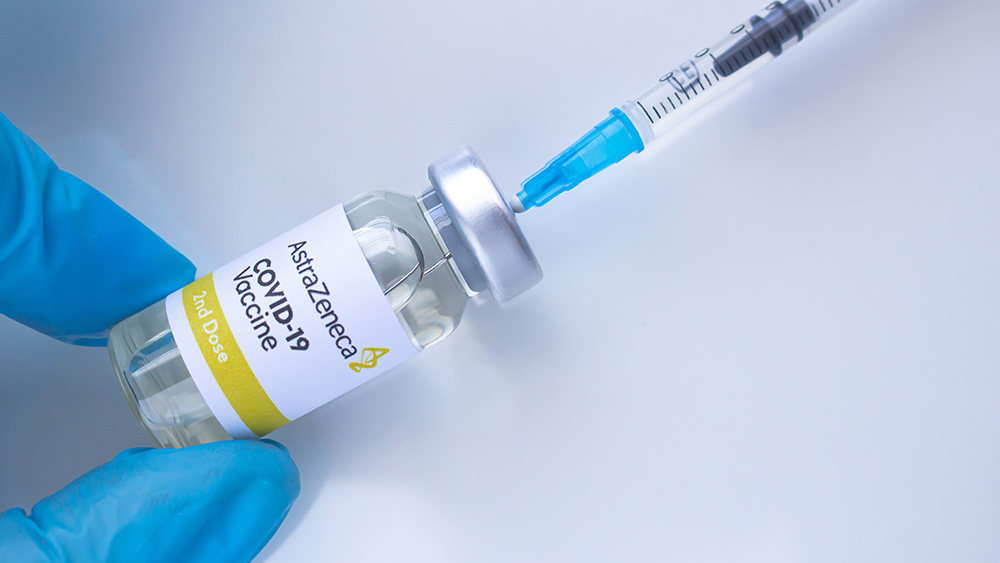
The pharmaceuticals firm recently conducted a late-stage trial in the United States involving more than 32,000 volunteers. Efficacy data from the trial showed that the vaccine is 79 percent effective at preventing symptomatic COVID-19 and 100 percent effective at preventing severe illness and hospitalization, the firm announced on Monday, March 22.
Later that day, the DSMB notified the National Institutes of Allergy and Infectious Disease (NIAID), the Biomedical Advanced Research and Development Authority and AstraZeneca that the firm might have included outdated information.
The NIAID said in a statement the next day that if AstraZeneca did submit outdated results, then it "may have provided an incomplete view of the efficacy data." The institute urged the company to work with DSMB to review its efficacy data and ensure that accurate, up-to-date information would be made public as quickly as possible.
"Outdated" data is preliminary, AstraZeneca claims
AstraZeneca issued a response to NIAID on Tuesday, March 23, saying that the numbers they released on Monday were based on a "pre-specified" interim analysis that had a data cutoff of Feb. 17. An interim analysis is a preliminary assessment that examines data from an ongoing trial.
"We have reviewed the preliminary assessment of the primary analysis and the results were consistent with the interim analysis," the pharmaceuticals giant said. "We will immediately engage with the independent data safety monitoring board (DSMB) to share our primary analysis with the most up to date efficacy data."
The firm added that it would release the results of the primary analysis in the next two days.
The AstraZeneca-Oxford vaccine has not yet been approved for emergency use in the United States but has been approved in several other countries. Many of those nations, including Germany, France, Spain and Italy, suspended the use of the vaccine over the past week due to reports that people who received it developed blood clots. (Related: Eight European nations pause AstraZeneca coronavirus vaccinations after reports of "serious" blood clot.)
Blood clots that form in the arms, legs or elsewhere can break free and move to the brain, heart or lungs, causing stroke, heart attack or other deadly blockages.
In Denmark, a 60-year-old woman who had been inoculated with the jab died due to blood clots. Health authorities said that the woman had a low number of blood platelets, clots in her small and large blood vessels and internal bleeding. Similar cases were also reported in Norway and in the E.U. European Medicines Agency's (EMA) database of suspected drug side effects.
Blood clot link cannot be ruled out yet
On March 18, the EMA declared that the vaccine is safe and effective at preventing COVID-19 and that the benefits of using it outweigh its risks. However, the regulatory body added that it could not definitively rule out a link at the moment.
EMA Executive Director Emer Cooke explained that the agency's safety committee found a limited number of blood clot cases that require further study. Committee Chair Dr. Sabine Straus added that it remained "premature to conclude" whether the blood clots were linked to a greater risk among groups or the makeup of the populations receiving the shot.
As of last week, roughly 18 million in Europe were vaccinated with the jab, Cooke said. Most of the blood clot cases were among women under the age of 55. The EMA said it would continue to assess reports and recommended adding a description of past blood clot cases to vaccine leaflets to raise public awareness.
Visit VaccineDamage.news to learn more about the dangers of the unproven COVID-19 vaccines.
Sources include:
Please contact us for more information.