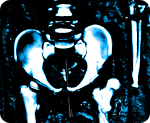
Exposed: Johnson & Johnson hid data showing 40 percent failure rate of hip implants
Thursday, January 31, 2013 by: Ethan A. Huff, staff writer
Tags: Johnson & Johnson, hip implants, failure
- Ginger over chemo: This powerful root can eliminate 10,000x the number of cancer cells
- Vaccine horrors: Medical mutilation of innocent children exposed in GRAPHIC photos of "safe" vaccines gone horribly wrong
- The REAL FAKE NEWS exposed: '97% of scientists agree on climate change' is an engineered hoax... here's what the media never told you
- Flu shot hoax admitted: "No controlled trials demonstrating a decrease in influenza"
- Suppressed science: Garlic proven to kill brain cancer cells without side effects
- Vaccine fraud exposed: Measles and mumps making a huge comeback because vaccines are designed to fail, say Merck virologists
- Cannabis kicks Lyme disease to the curb
- Vaccine industry in panic over global effort to remove all mercury from vaccines
- Amazon Echo is the ultimate spy device that records everything you say
- Breast Cancer Fund exposes the cancer-causing chemicals lurking in food
- Natural Treatments Shrink and Eliminate Fibroid Tumors
- Tungsten in rice protein: Health Ranger releases new chart, petitions all protein manufacturers to commit to reasonable industry standards for heavy metals
- Biotech company needlessly uses aborted human fetal cells to test artificial food flavors
- Are you detoxing, or RE-toxing? Zeolite consumers urinate out the same metals found in the zeolites they just swallowed... then they repeat daily
- How to Prevent and Eliminate Fibroids Naturally
- Measles vaccine far more dangerous than measles itself
- The United Nations 2030 Agenda decoded: It's a blueprint for the global enslavement of humanity under the boot of corporate masters
- Antibiotics used in meat pose a threat to public health, admits FDA
- Supplements to detox the body from vaccinations
- Beat cancer with 35% hydrogen peroxide
- The REAL FAKE NEWS exposed: '97% of scientists agree on climate change' is an engineered hoax... here's what the media never told you
- Ginger over chemo: This powerful root can eliminate 10,000x the number of cancer cells
- Vaccine fraud exposed: Measles and mumps making a huge comeback because vaccines are designed to fail, say Merck virologists
- Vaccine horrors: Medical mutilation of innocent children exposed in GRAPHIC photos of "safe" vaccines gone horribly wrong
- 10 easy, natural remedies for conquering the common cold
- Forget Filling Cavities: Regrow Your Teeth Instead
- The best and worst forms of magnesium to take as a supplement
- Schizophrenia Not Caused by Genes, Scientist Says
- Flu shot hoax admitted: "No controlled trials demonstrating a decrease in influenza"
- Apricot Seeds Kill Cancer Cells without Side Effects
- Biologists say Monsanto's glyphosate is devastating wildlife; deer populations down 50 percent
- Kill internal parasites easily with these 8 herbs
- INVESTIGATION: Three days before Dr. Bradstreet was found dead in a river, U.S. govt. agents raided his research facility to seize a breakthrough cancer treatment called GcMAF
- Tungsten in rice protein: Health Ranger releases new chart, petitions all protein manufacturers to commit to reasonable industry standards for heavy metals
- The United Nations 2030 Agenda decoded: It's a blueprint for the global enslavement of humanity under the boot of corporate masters
- Wheat crops soaked with glyphosate weedkiller before harvest... Are you eating 'cancer bread'?
- The REAL FAKE NEWS exposed: '97% of scientists agree on climate change' is an engineered hoax... here's what the media never told you
- Beat cancer with 35% hydrogen peroxide
- Supplements to detox the body from vaccinations
- Vaccine fraud exposed: Measles and mumps making a huge comeback because vaccines are designed to fail, say Merck virologists
- The best and worst forms of magnesium to take as a supplement
- The United Nations 2030 Agenda decoded: It's a blueprint for the global enslavement of humanity under the boot of corporate masters
- Forget Filling Cavities: Regrow Your Teeth Instead
- Bullet proof immune system - how to not get a cold ever
- What's really in vaccines? Proof of MSG, formaldehyde, aluminum and mercury
- Measles vaccine far more dangerous than measles itself
- Six little-known natural remedies for tinnitus
- Apricot Seeds Kill Cancer Cells without Side Effects
- INVESTIGATION: Three days before Dr. Bradstreet was found dead in a river, U.S. govt. agents raided his research facility to seize a breakthrough cancer treatment called GcMAF
- Schizophrenia Not Caused by Genes, Scientist Says
- Ginger over chemo: This powerful root can eliminate 10,000x the number of cancer cells
- Dandelion root far more effective in fighting cancer cells than chemotherapy
- Aajonus Vonderplanitz PhD, key informant in prosecution of Sharon Palmer and James Stewart, found to have faked academic credentials
- Vaccine horrors: Medical mutilation of innocent children exposed in GRAPHIC photos of "safe" vaccines gone horribly wrong
- Newly released JFK files reveal Pentagon's role in creating Lyme disease and covid in the same lab
- Eleven days before Iran bombed Tel Aviv, my microscope revealed haunting images of EXACTLY what would happen
- Morphic resonance “remote viewing” reveals iconic Middle East images of stealth bombers, a falcon and a one-horned ram
- DECENTRALIZED SPIRITUALITY and the true teachings of Christ: Overcoming the censorship, threats and lies of organized religion to truly know God and the Universal Christ
- HEALTH SECRETS: How to Instantly Block MSG Toxicity Using Natural Substances (and the secret of Methylene Blue)
- Mike Adams releases country western hit single: Goin’ Back in Time is Comin’ Home
- The Health Ranger releases “Vaccine Zombie” song and music video, using AI-animated zombies for the music video
- EPA advisor admits the agency is funneling billions to climate groups ahead of Trump’s return to White House
- BOMBSHELL: Internal Pfizer documents exposed and reveal at least 16 PERCENT of their mRNA vaccine "adverse events" are REPRODUCTIVE DISORDERS
- Amazing microscopy photos reveal how freezing crystals attempt to mimic electronic structures they are touching
- The Coming Gold Revaluation: Strategic Financial Realignment in an Era of Dollar Collapse
- The AI Data Center Wars Have Begun… Farms, Water and Electricity is Stripped from Humans to Power the Machines
- Urgent Wake-Up Call: The Coming AI Robot Wars and the Great Human Unity
- The War on Light: How Governments and Big Pharma Keep You Sick By Blocking Healing Photons
- BOMBSHELL: DNA testing kits are a SCAM to develop ethnic-specific bioweapons
- Aerosolized bioweapons? Strange “diploid biomasses” falling out of the sky in Florida captured under the microscope
- BOMBSHELL: Covid-19 mRNA nanoparticles EMIT LIGHT SIGNALS that communicate MAC addresses used for self-assembly inside the blood vessels
- Dr. Mike Yeadon releases 15-minute testimony - WATCH - about genocidal intent of COVID “vaccines”
As reported by The New York Times (NYT), the device in question, known as an Articular Surface Replacement (ASR), was first introduced back in the early 2000s as an alternative to traditional hip implant devices. Its design was supposed to overcome deficiencies inherent in previous models that were made from metal and plastic parts. But a major design flaw in the ASR caused the device's supposedly innovative ball mechanism to grind against the inside cup, which in turn caused it to release metallic debris inside the bodies of many patients who received the implant.
Just a few years after its release by J&J's DePuy Orthopaedics unit, internal tests conducted by company engineers revealed that the device was a complete failure, and that it was unsuitable for continued use. But rather than address the issue and recall the device, J&J continued to market the faulty ASR to surgeons across the country for at least another three years, even as thousands of these same physicians were rejecting the device in favor of safer and more durable alternatives.
"The ASR represents one of the biggest medical device failures in recent decades," writes Barry Meier for NYT about the fiasco. "According to DePuy's internal estimates, it is projected to fail within five years in about 40 percent of patients who received one. That is eight times the failure rate of most orthopedic implants."
J&J eventually issued a recall on the ASR in 2010, but nearly 100,000 patients worldwide had already received the device, and many of these patients are now suing the company for fraud. J&J also failed to admit there was anything wrong with the device during the recall, insisting instead that "poor sales" were responsible for its discontinuation. However, internal records have now revealed that DePuy executives, including company president Andrew Ekdahl, were fully aware of the dangers associated with the ASR and yet did nothing about them.
Patients injured as a result of the faulty implant are being represented by the national law firm Parker Walchman LLP. Both the standard ASR and the resurfacing ASR manufactured by J&J DePuy are included in the lawsuit.
Sources for this article include:
http://www.nytimes.com
http://www.prweb.com
Johnson & Johnson at FETCH.news
Get independent news alerts on natural cures, food lab tests, cannabis medicine, science, robotics, drones, privacy and more.
Take Action: Support Natural News by linking to this article from your website
Permalink to this article:
Embed article link: (copy HTML code below):
Reprinting this article:
Non-commercial use OK, cite NaturalNews.com with clickable link.
Follow Natural News on Facebook, Twitter, Google Plus, and Pinterest
Science News & Studies
Medicine News and Information
Food News & Studies
Health News & Studies
Herbs News & Information
Pollution News & Studies
Cancer News & Studies
Climate News & Studies
Survival News & Information
Gear News & Information
News covering technology, stocks, hackers, and more


"Big Tech and mainstream media are constantly trying to silence the independent voices that dare to bring you the truth about toxic food ingredients, dangerous medications and the failed, fraudulent science of the profit-driven medical establishment.
Email is one of the best ways to make sure you stay informed, without the censorship of the tech giants (Google, Apple, Facebook, Twitter, YouTube, etc.). Stay informed and you'll even likely learn information that may help save your own life."
–The Health Ranger, Mike Adams























